From a location that will remain secret for now.
Every so often I get out of my hospital bed and look at some rocks. This image comes from a rock slab I took down to the SEM lab in the Geology Department at the University of Toronto last month some time.
Most people have some idea of how a scanning electron microscope works. Fewer will know about an attachment that many of them have--an energy-dispersive X-ray analysis detector. This device allows for estimation of the elemental composition of a point on a mineral (or a section of whole rock) in a non-destructive fashion.
Blasting a sample with electrons (how the SEM works) ionizes the target area--when it recaptures an electron it releases a quantum of energy in the X-ray spectrum that is characteristic of the element that has been ionized. This allows for the specific elements to be identified and their relative amount quantified.
A second method of analysis involves back-scattered electrons, which create a grey-scale image that results is grey scale, with lighter coloured grains containing heavier elements.
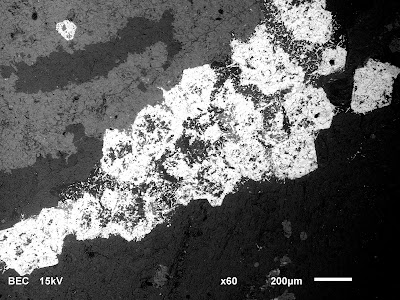
The backscattered electron image above tells me that the white grains have the heaviest elements in them. The X-ray spectrum (not shown) tells me that the heavy element present is niobium. The mineralogy work previously done suggested that the principal niobium-bearing mineral present is pyrochlore--but the x-ray spectrum suggests that many of these grains are actually ferro-columbite (as columbite, but Fe>>Nb), pseudomorphed from pyrochlore.
The gangue minerals are represented by two different regions of grey. The light grey can be shown to be phosphates (mainly apatite) and the darker grey is carbonate (mainly dolomite).
The image above captures something interesting. The columbite grains have begun to lose their distinction from the gangue minerals. Niobium and other heavy elements are being shifted around (the bright white "tendrils" surrounded by black [=silica]) by some late-stage fluid reaction. The x-ray spectrum shows that in addition to Nb, there are rare earth elements present (mainly cerium). This suggests alteration of the columbite to possibly fersmite--although I haven't isolated grains well enough to establish the crystal structure yet.
I wanted to share this because I think this is a spectacular image--from a scientific perspective. From a metallurgical perspective, we would prefer not to see all of those little unrecoverable tendrils of Nb and Ce locked in silica.
Every so often I get out of my hospital bed and look at some rocks. This image comes from a rock slab I took down to the SEM lab in the Geology Department at the University of Toronto last month some time.
Most people have some idea of how a scanning electron microscope works. Fewer will know about an attachment that many of them have--an energy-dispersive X-ray analysis detector. This device allows for estimation of the elemental composition of a point on a mineral (or a section of whole rock) in a non-destructive fashion.
Blasting a sample with electrons (how the SEM works) ionizes the target area--when it recaptures an electron it releases a quantum of energy in the X-ray spectrum that is characteristic of the element that has been ionized. This allows for the specific elements to be identified and their relative amount quantified.
A second method of analysis involves back-scattered electrons, which create a grey-scale image that results is grey scale, with lighter coloured grains containing heavier elements.
The backscattered electron image above tells me that the white grains have the heaviest elements in them. The X-ray spectrum (not shown) tells me that the heavy element present is niobium. The mineralogy work previously done suggested that the principal niobium-bearing mineral present is pyrochlore--but the x-ray spectrum suggests that many of these grains are actually ferro-columbite (as columbite, but Fe>>Nb), pseudomorphed from pyrochlore.
The gangue minerals are represented by two different regions of grey. The light grey can be shown to be phosphates (mainly apatite) and the darker grey is carbonate (mainly dolomite).
The image above captures something interesting. The columbite grains have begun to lose their distinction from the gangue minerals. Niobium and other heavy elements are being shifted around (the bright white "tendrils" surrounded by black [=silica]) by some late-stage fluid reaction. The x-ray spectrum shows that in addition to Nb, there are rare earth elements present (mainly cerium). This suggests alteration of the columbite to possibly fersmite--although I haven't isolated grains well enough to establish the crystal structure yet.
I wanted to share this because I think this is a spectacular image--from a scientific perspective. From a metallurgical perspective, we would prefer not to see all of those little unrecoverable tendrils of Nb and Ce locked in silica.
No comments:
Post a Comment